Expo
view channel
view channel
view channel
view channel
Medical Imaging
AICritical CareSurgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Webinars

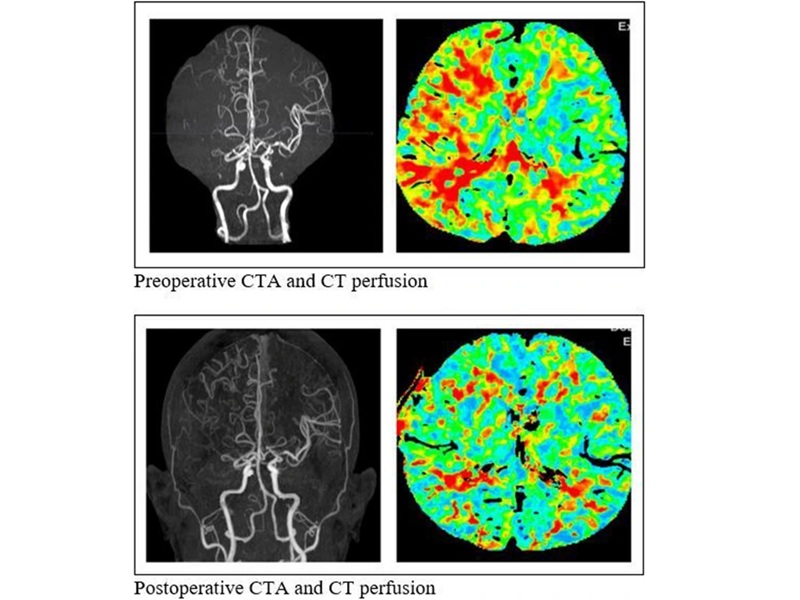
- Remote Ventilate View Platform Enables Real-Time Monitoring of Patient-Ventilator Asynchrony
- Soft “Cyborg” Cardiac Patches Improve Stem Cell Heart Repair
- Soft Wearable System Offers Continuous Wireless Monitoring of Neonatal Health
- AI-Enhanced Wearables Could Transform Type 2 Diabetes and Prediabetes Care
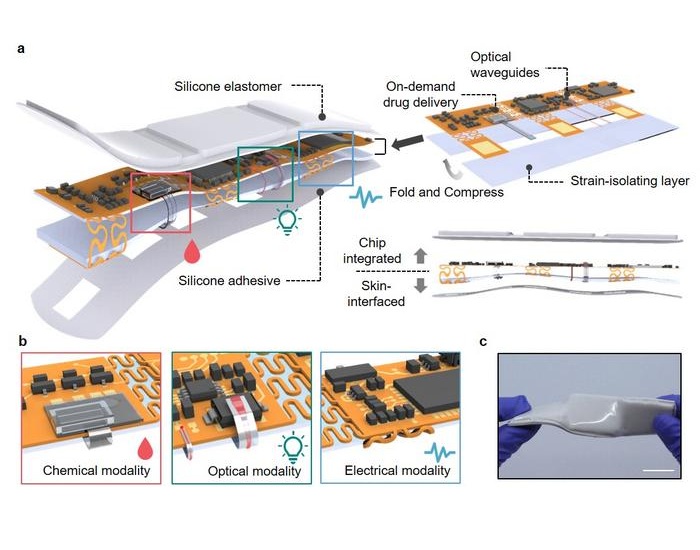
- Breathable Electronic Skin Paves Way for Next-Generation Wearable Devices
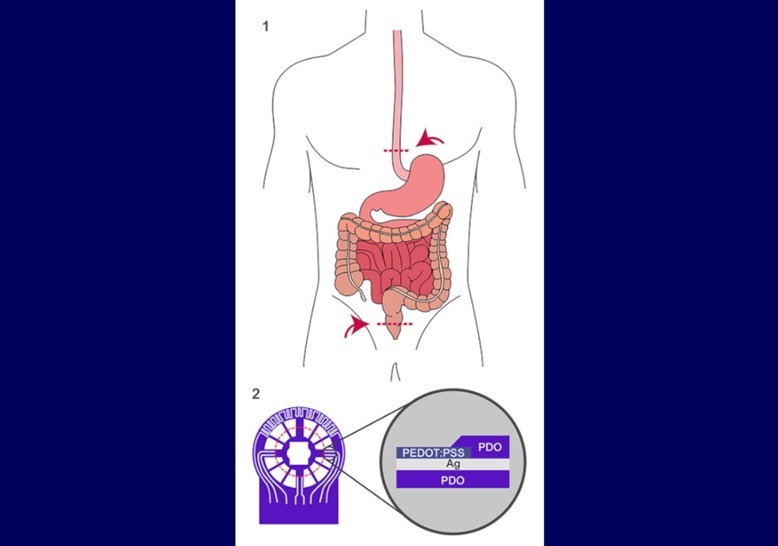
- Implantable Absorbable Sensor Detects Life-Threatening Complications After Intestinal Surgery
- New Study Findings Enable Improved Ventilation During Complex Lung Surgery
- 3D-Printed Blood Vessel Scaffolds Could Transform Heart Bypass Surgeries
- Novel Imaging Technique Helps View Blood Perfusion During Esophageal Surgery
- Minimally Invasive Surgery Proven Safe and Effective for Complex ‘Whipple’ Procedure
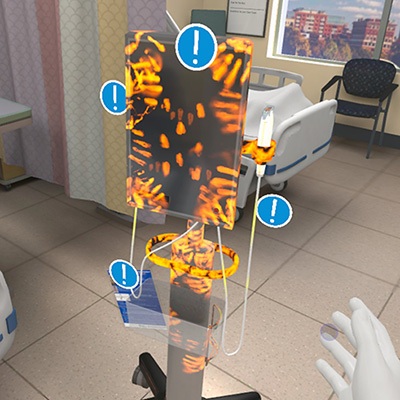
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- B. Braun Acquires Digital Microsurgery Company True Digital Surgery
- CMEF 2025 to Promote Holistic and High-Quality Development of Medical and Health Industry
- Bayer and Broad Institute Extend Research Collaboration to Develop New Cardiovascular Therapies
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
- Expanded Collaboration to Transform OR Technology Through AI and Automation

 Expo
Expo
- Remote Ventilate View Platform Enables Real-Time Monitoring of Patient-Ventilator Asynchrony
- Soft “Cyborg” Cardiac Patches Improve Stem Cell Heart Repair
- Soft Wearable System Offers Continuous Wireless Monitoring of Neonatal Health
- AI-Enhanced Wearables Could Transform Type 2 Diabetes and Prediabetes Care
- Breathable Electronic Skin Paves Way for Next-Generation Wearable Devices
- Implantable Absorbable Sensor Detects Life-Threatening Complications After Intestinal Surgery
- New Study Findings Enable Improved Ventilation During Complex Lung Surgery
- 3D-Printed Blood Vessel Scaffolds Could Transform Heart Bypass Surgeries
- Novel Imaging Technique Helps View Blood Perfusion During Esophageal Surgery
- Minimally Invasive Surgery Proven Safe and Effective for Complex ‘Whipple’ Procedure
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- B. Braun Acquires Digital Microsurgery Company True Digital Surgery
- CMEF 2025 to Promote Holistic and High-Quality Development of Medical and Health Industry
- Bayer and Broad Institute Extend Research Collaboration to Develop New Cardiovascular Therapies
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
- Expanded Collaboration to Transform OR Technology Through AI and Automation























_1.jpg)