Expo
view channel
view channel
view channel
Medical Imaging
AICritical CareSurgical TechniquesPatient CareHealth IT
Business
Events
Webinars

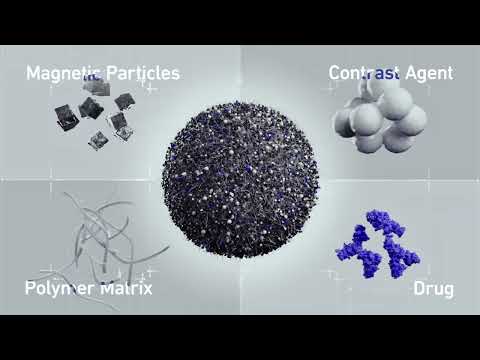
- Bioadhesive Patch Eliminates Cancer Cells That Remain After Brain Tumor Surgery
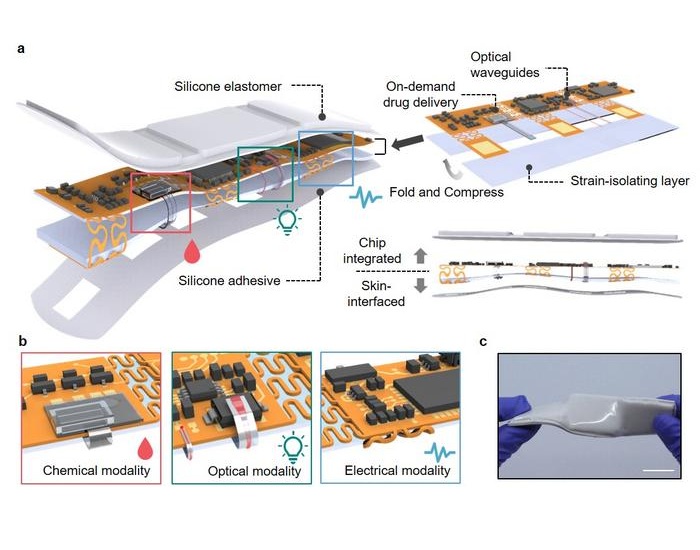
- Wearable Patch Provides Up-To-The-Minute Readouts of Medication Levels in Body
- Living Implant Could End Daily Insulin Injections
- New Spray-Mist Device Delivers Antibiotics Directly into Infected Tissue
- Intelligent Camera System Continuously Monitors Premature Babies in NICU
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
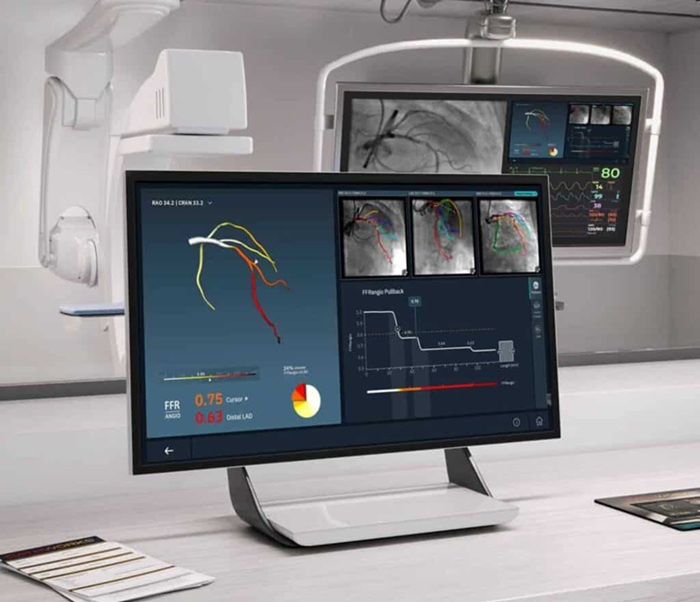
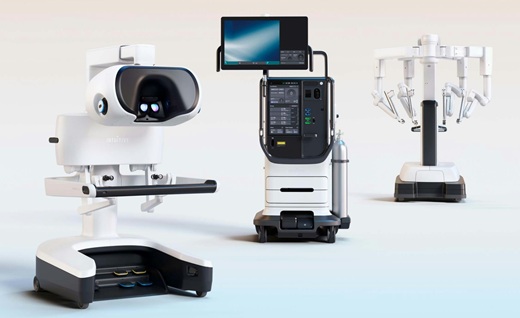
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market

 Expo
Expo
- Bioadhesive Patch Eliminates Cancer Cells That Remain After Brain Tumor Surgery
- Wearable Patch Provides Up-To-The-Minute Readouts of Medication Levels in Body
- Living Implant Could End Daily Insulin Injections
- New Spray-Mist Device Delivers Antibiotics Directly into Infected Tissue
- Intelligent Camera System Continuously Monitors Premature Babies in NICU
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market