Expo
view channel
view channel
view channel
view channel
Medical Imaging
AICritical CareSurgical TechniquesPatient Care
Point of CareBusiness
Events
Webinars

- Smart T-Shirt Uses AI to Enhance Detection of Heart Rhythm Disorders
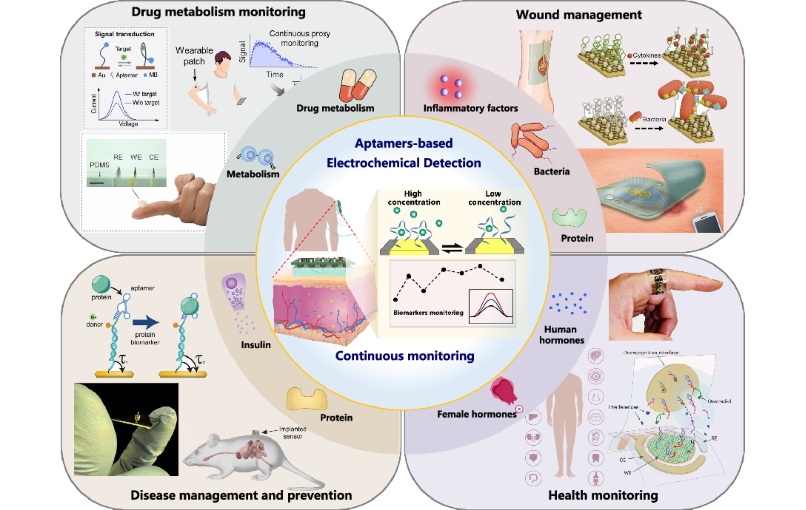
- Aptamers Enable Real-Time Biomarker Tracking Without Blood Draws
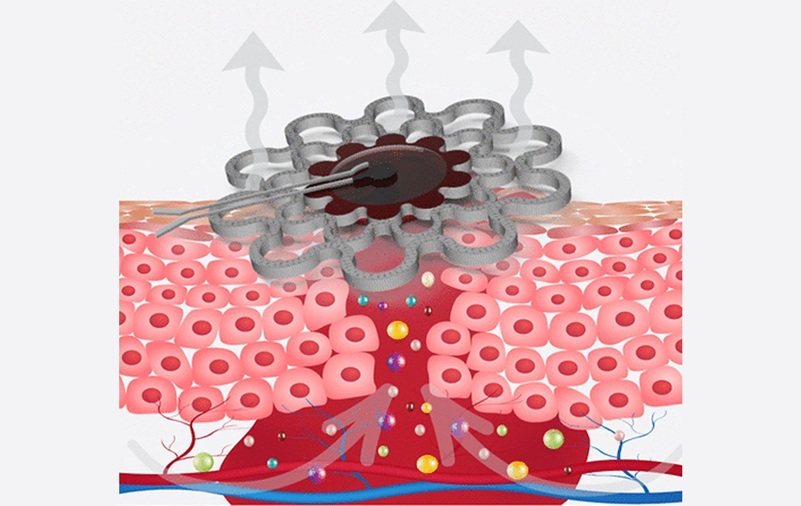
- Specialized Dressing with Sensor Monitors pH Levels in Chronic Wounds
- AI Model Could Help Diagnose Spinal Cord Disease Up To 30 Months Earlier
- 3D-Printed Swallowable Robot Could Perform Gastrointestinal Procedures
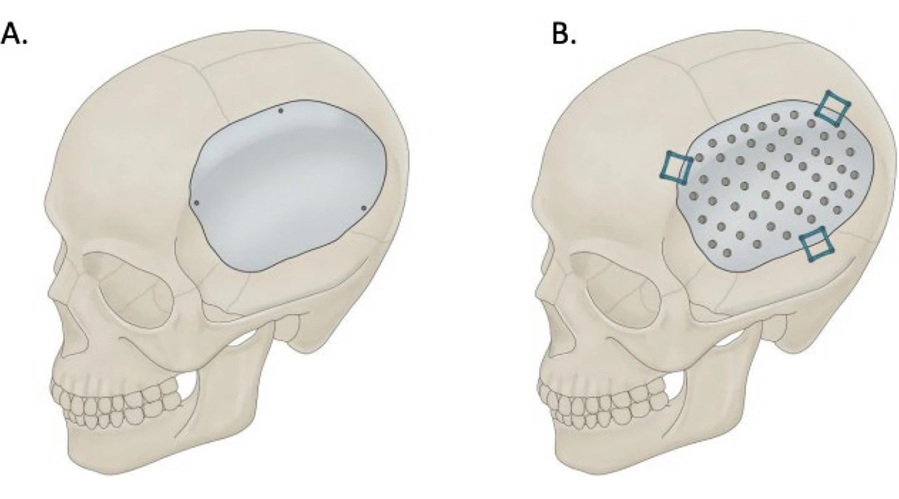
- Redesigned Surgical Laser Cuts Bone Deeper and Faster Than Before
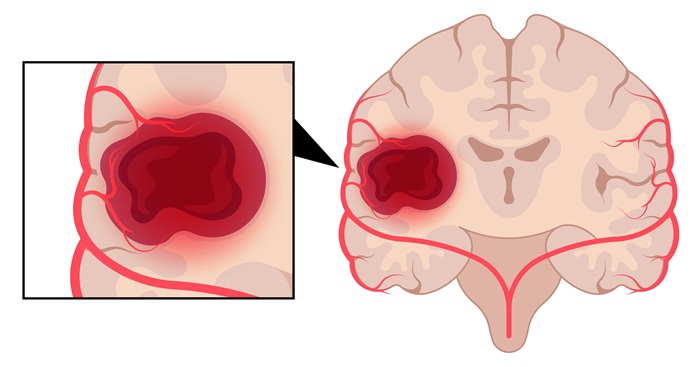
- Laser Ablation Plus Immunotherapy Improves Survival in Recurrent Glioblastoma
- New Method Offers Less Invasive Detection of Susceptibility to Rare Anesthesia Reaction
- Brain Implant Records Neural Signals and Delivers Precise Medication
- AI-Based OCT Image Analysis Identifies High-Risk Plaques in Coronary Arteries
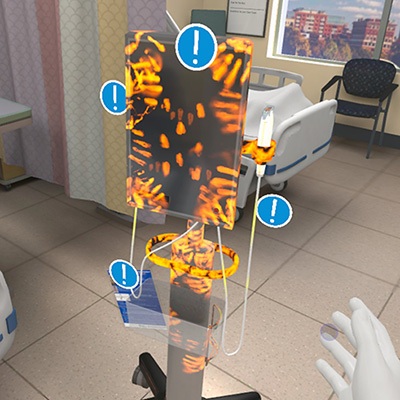
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market

 Expo
Expo
- Smart T-Shirt Uses AI to Enhance Detection of Heart Rhythm Disorders
- Aptamers Enable Real-Time Biomarker Tracking Without Blood Draws
- Specialized Dressing with Sensor Monitors pH Levels in Chronic Wounds
- AI Model Could Help Diagnose Spinal Cord Disease Up To 30 Months Earlier
- 3D-Printed Swallowable Robot Could Perform Gastrointestinal Procedures
- Redesigned Surgical Laser Cuts Bone Deeper and Faster Than Before
- Laser Ablation Plus Immunotherapy Improves Survival in Recurrent Glioblastoma
- New Method Offers Less Invasive Detection of Susceptibility to Rare Anesthesia Reaction
- Brain Implant Records Neural Signals and Delivers Precise Medication
- AI-Based OCT Image Analysis Identifies High-Risk Plaques in Coronary Arteries
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market