Expo
view channel
view channel
view channel
view channel
Medical Imaging
AICritical Care
Patient CareHealth ITPoint of CareBusiness
Events
Webinars

- New Inhalable Treatment for TB Lowers Side Effects
- AI Algorithm Improves Antibiotic Decision-Making in Urinary Tract Infection
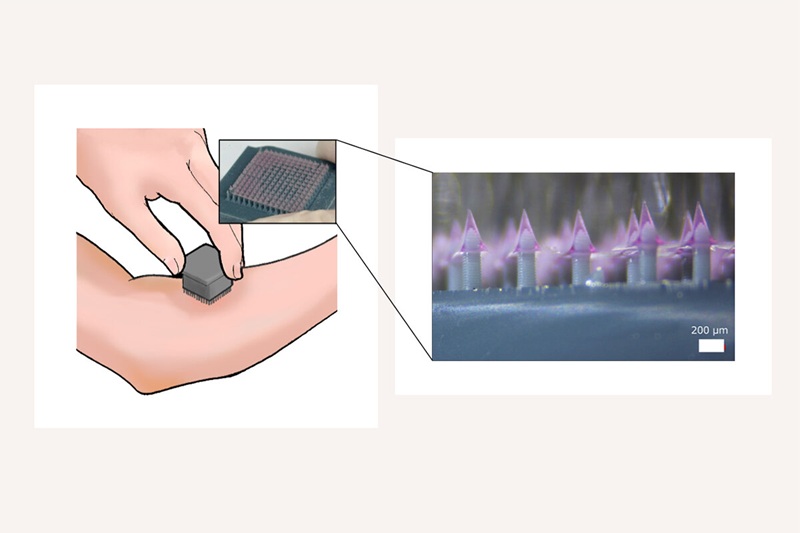
- 3D-Printed System Enhances Vaccine Delivery Via Microneedle Array Patch
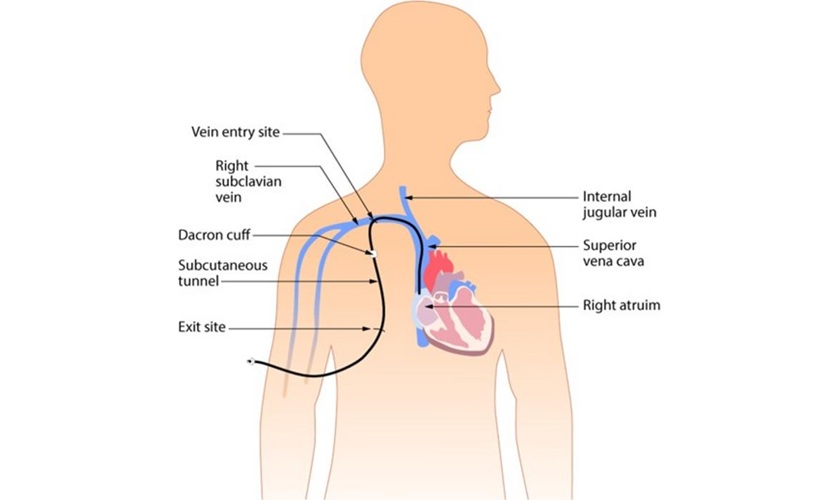
- Whole-Heart Mapping Technology Provides Comprehensive Real-Time View of Arrhythmias
- Wearable Device for Diabetics Could Replace Continuous Glucose Monitoring Systems
- Neural Device Regrows Surrounding Skull After Brain Implantation
- Surgical Innovation Cuts Ovarian Cancer Risk by 80%
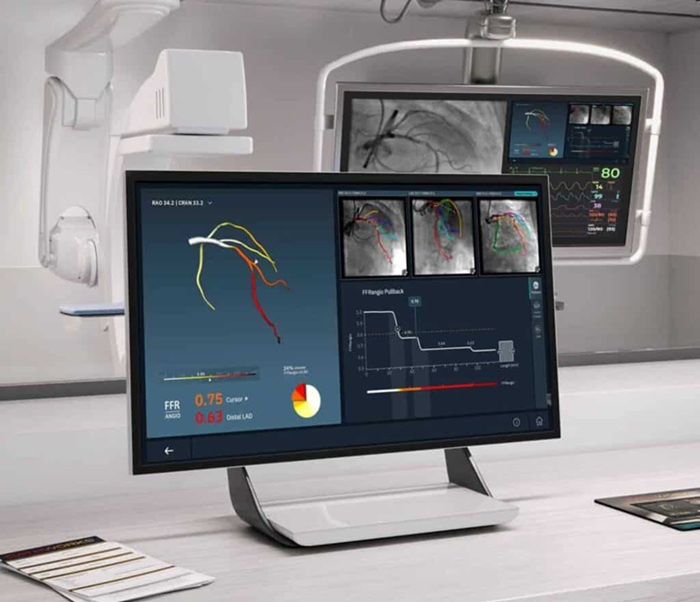
- New Imaging Combo Offers Hope for High-Risk Heart Patients
- New Classification System Brings Clarity to Brain Tumor Surgery Decisions
- Boengineered Tissue Offers New Hope for Secondary Lymphedema Treatment
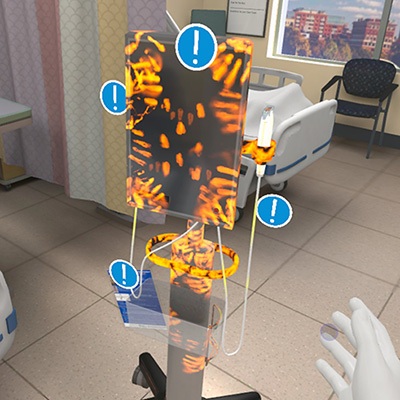
- VR Training Tool Combats Contamination of Portable Medical Equipment
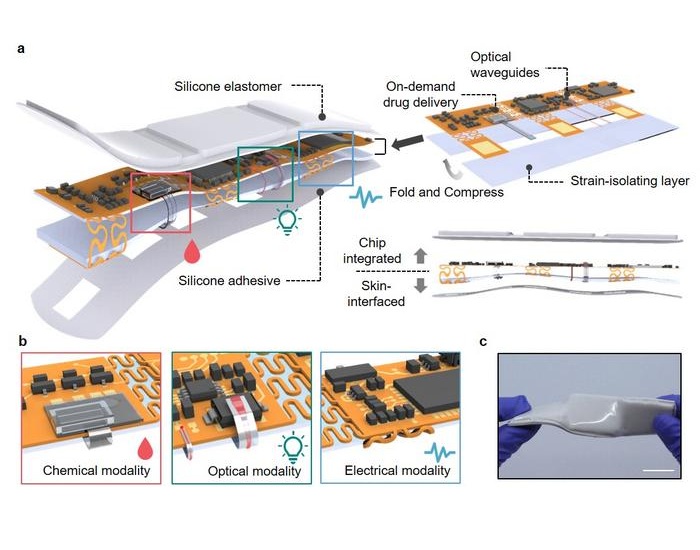
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
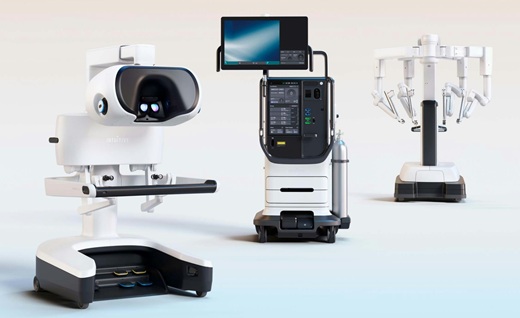
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market

 Expo
Expo
- New Inhalable Treatment for TB Lowers Side Effects
- AI Algorithm Improves Antibiotic Decision-Making in Urinary Tract Infection
- 3D-Printed System Enhances Vaccine Delivery Via Microneedle Array Patch
- Whole-Heart Mapping Technology Provides Comprehensive Real-Time View of Arrhythmias
- Wearable Device for Diabetics Could Replace Continuous Glucose Monitoring Systems
- Neural Device Regrows Surrounding Skull After Brain Implantation
- Surgical Innovation Cuts Ovarian Cancer Risk by 80%
- New Imaging Combo Offers Hope for High-Risk Heart Patients
- New Classification System Brings Clarity to Brain Tumor Surgery Decisions
- Boengineered Tissue Offers New Hope for Secondary Lymphedema Treatment
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market















.jpg)