Expo
view channel
view channel
view channel
view channel
Medical Imaging
AICritical CareSurgical TechniquesPatient CareHealth ITPoint of Care
Events
Webinars

- AI Risk Prediction Tool Improves Treatment of Cancer Patients after Heart Attack
- Glowing Bacterial Sensors Could Improve Detection of Gut Illness
- Gut Bacteria from Amphibians and Reptiles Show Complete Tumor Elimination
- Innovative ‘Poop Pills’ Dramatically Improve Cancer Treatment
- High-Dose Inhaled Nitric Oxide Emerges as Promising Antimicrobial Therapy
- New Imaging Combo Offers Hope for High-Risk Heart Patients
- New Classification System Brings Clarity to Brain Tumor Surgery Decisions
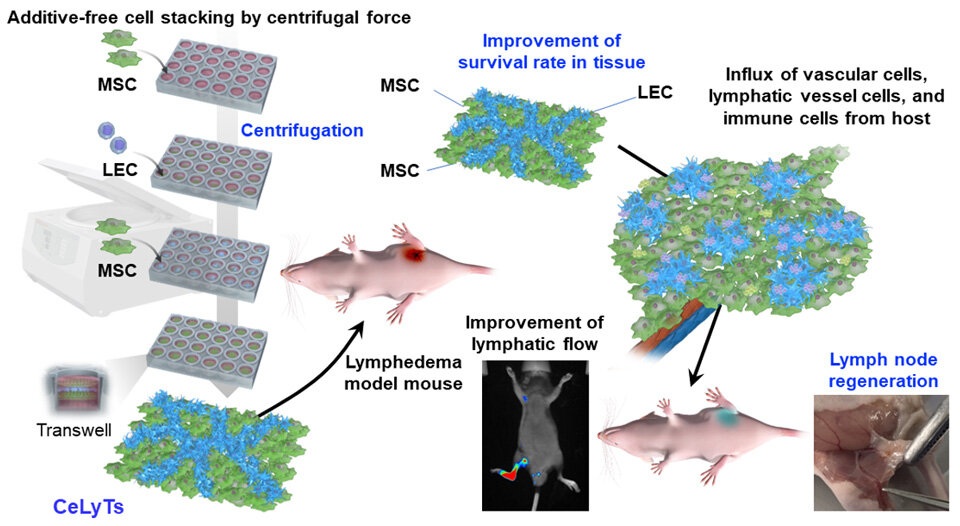
- Boengineered Tissue Offers New Hope for Secondary Lymphedema Treatment
- New AI Approach to Improve Surgical Imaging
- Dual-Energy Catheter Brings New Flexibility to AFib Ablation
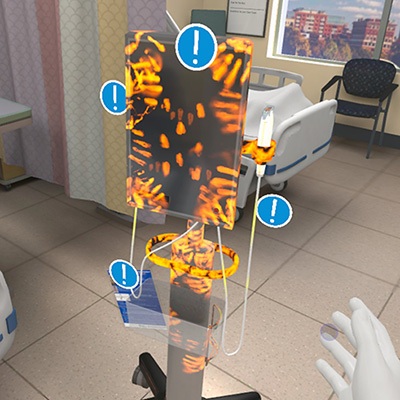
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market

 Expo
Expo
- AI Risk Prediction Tool Improves Treatment of Cancer Patients after Heart Attack
- Glowing Bacterial Sensors Could Improve Detection of Gut Illness
- Gut Bacteria from Amphibians and Reptiles Show Complete Tumor Elimination
- Innovative ‘Poop Pills’ Dramatically Improve Cancer Treatment
- High-Dose Inhaled Nitric Oxide Emerges as Promising Antimicrobial Therapy
- New Imaging Combo Offers Hope for High-Risk Heart Patients
- New Classification System Brings Clarity to Brain Tumor Surgery Decisions
- Boengineered Tissue Offers New Hope for Secondary Lymphedema Treatment
- New AI Approach to Improve Surgical Imaging
- Dual-Energy Catheter Brings New Flexibility to AFib Ablation
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market














.jpg)