Expo
view channel
view channel
view channel
view channel
Medical Imaging
AICritical Care
Patient CareHealth ITPoint of CareBusiness
Events
Webinars

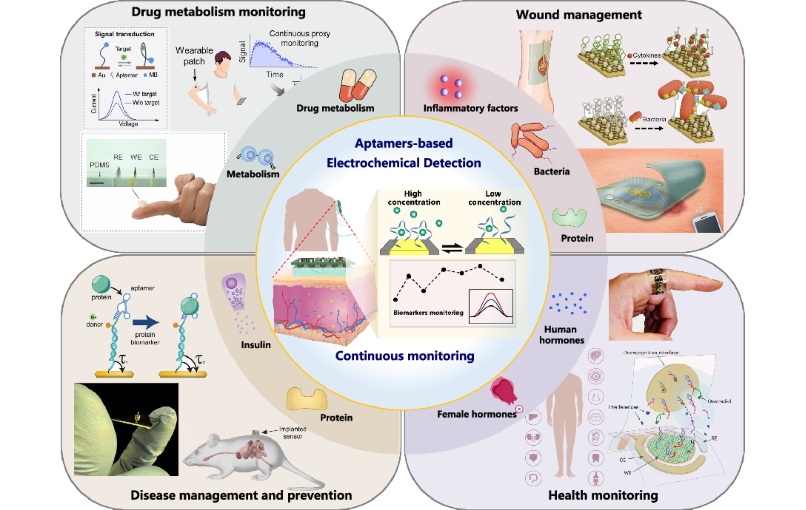
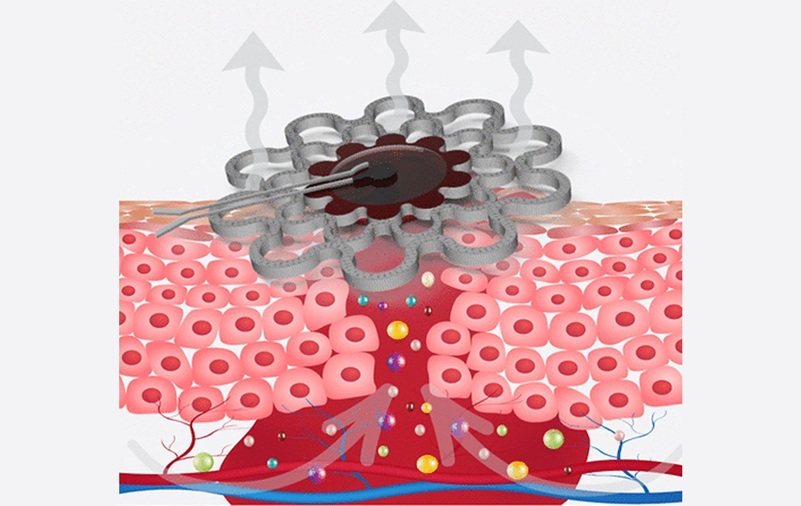
- Specialized Dressing with Sensor Monitors pH Levels in Chronic Wounds
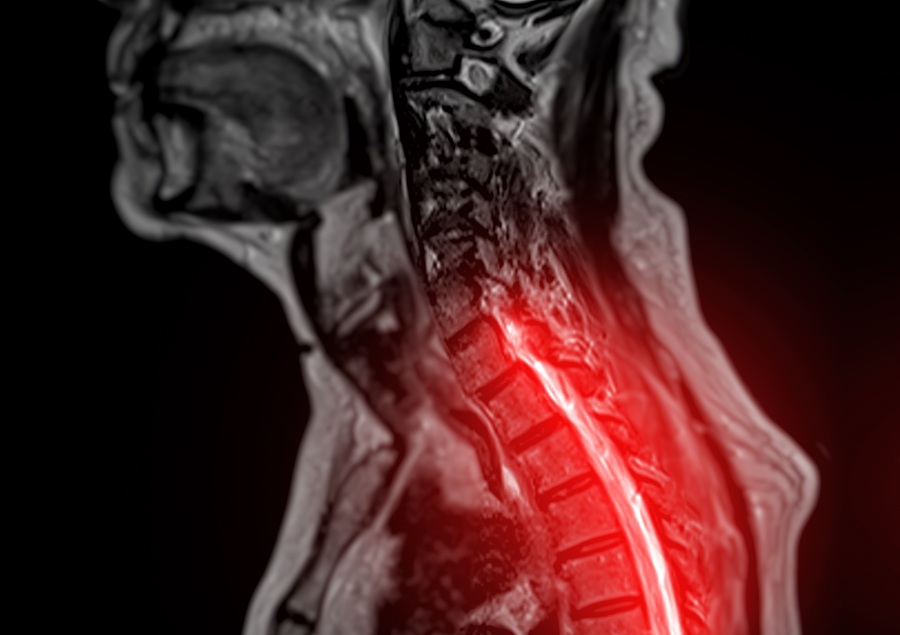
- AI Model Could Help Diagnose Spinal Cord Disease Up To 30 Months Earlier
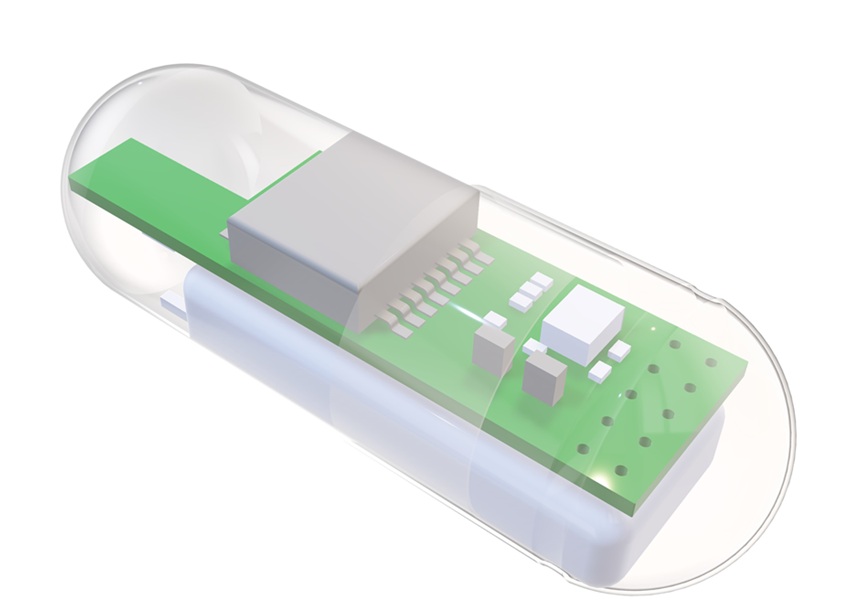
- 3D-Printed Swallowable Robot Could Perform Gastrointestinal Procedures
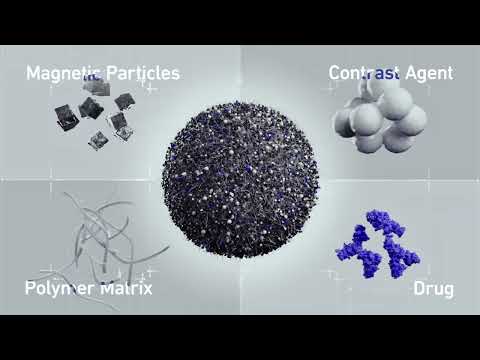
- Next-Gen Hydrogel Could Transform Soft Tissue and Organ Repair
- Engineered Cancer Eating Bacteria Consume Tumors from Inside Out
- AI-Based OCT Image Analysis Identifies High-Risk Plaques in Coronary Arteries
- Neural Device Regrows Surrounding Skull After Brain Implantation
- Surgical Innovation Cuts Ovarian Cancer Risk by 80%
- New Imaging Combo Offers Hope for High-Risk Heart Patients
- New Classification System Brings Clarity to Brain Tumor Surgery Decisions
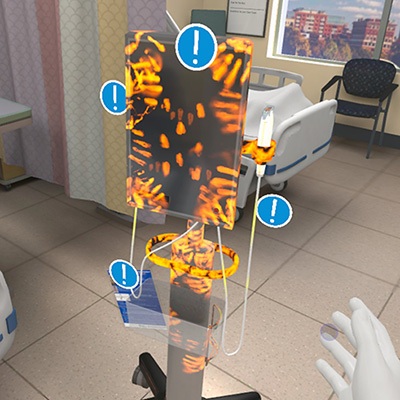
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market

 Expo
Expo
- Specialized Dressing with Sensor Monitors pH Levels in Chronic Wounds
- AI Model Could Help Diagnose Spinal Cord Disease Up To 30 Months Earlier
- 3D-Printed Swallowable Robot Could Perform Gastrointestinal Procedures
- Next-Gen Hydrogel Could Transform Soft Tissue and Organ Repair
- Engineered Cancer Eating Bacteria Consume Tumors from Inside Out
- AI-Based OCT Image Analysis Identifies High-Risk Plaques in Coronary Arteries
- Neural Device Regrows Surrounding Skull After Brain Implantation
- Surgical Innovation Cuts Ovarian Cancer Risk by 80%
- New Imaging Combo Offers Hope for High-Risk Heart Patients
- New Classification System Brings Clarity to Brain Tumor Surgery Decisions
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic and Mindray Expand Strategic Partnership to Ambulatory Surgery Centers in the U.S.
- FDA Clearance Expands Robotic Options for Minimally Invasive Heart Surgery
- WHX in Dubai (formerly Arab Health) to debut specialised Biotech & Life Sciences Zone as sector growth accelerates globally
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market