Expo
view channel
view channel
view channel
view channel
Medical Imaging
AICritical CareSurgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Webinars

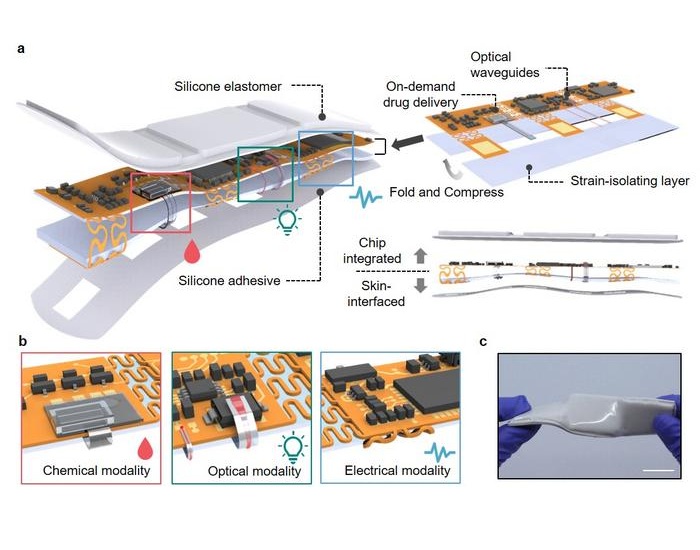
- Imaging Technology Detects Early Signs of Cardiovascular Risk Through Skin
- New Therapeutic Approach Marks Breakthrough in Pediatric Heart Disease
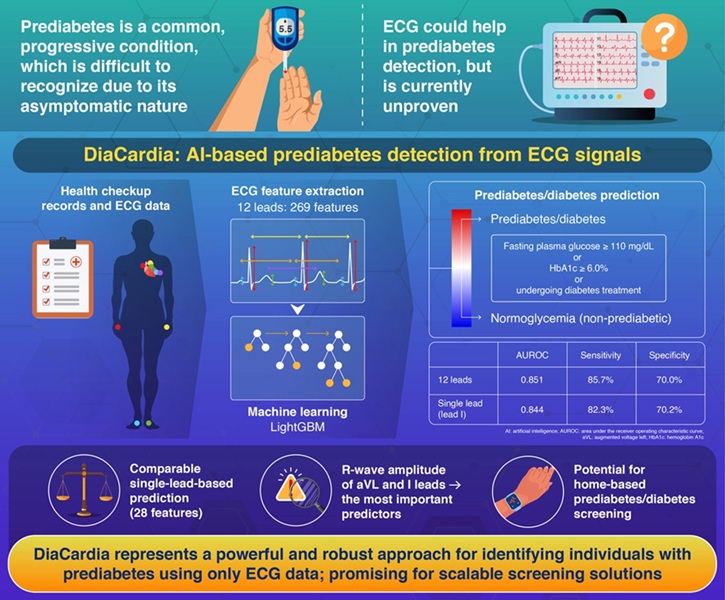
- AI Model Accurately Identifies Prediabetics Using Only ECG Data
- Injectable Disease-Fighting Nanorobots to Improve Precision Cancer Therapy
- Web-Based Tool Enables Early Detection and Prevention of Chronic Kidney Disease
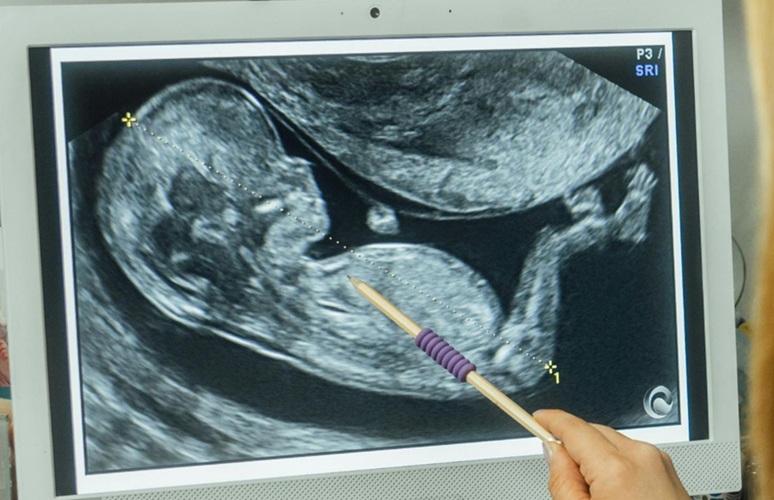
- First-Of-Its-Kind Probe Monitors Fetal Health in Utero During Surgery
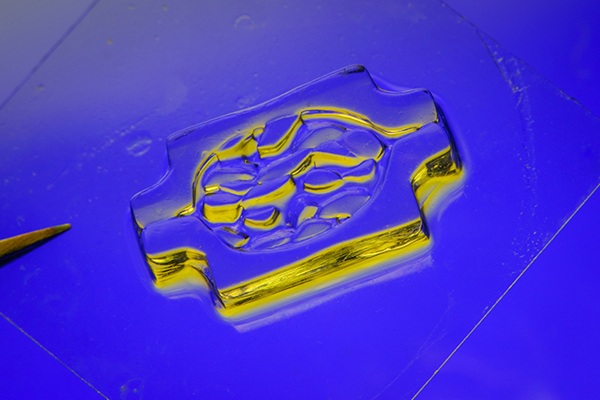
- Light-Activated Tissue Adhesive Patch Achieves Rapid and Watertight Neurosurgical Sealing
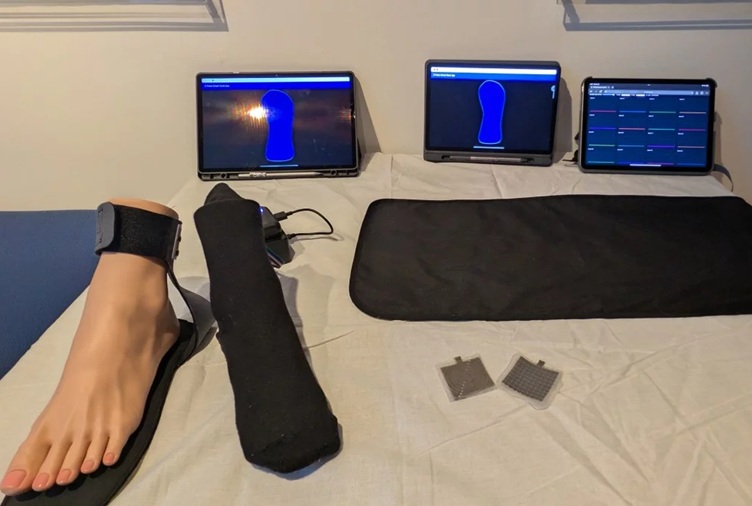
- Ultrasound Device Offers Non-Invasive Treatment for Kidney Stones
- Minimally Invasive Coronary Artery Bypass Method Offers Safer Alternative to Open-Heart Surgery
- Injectable Breast ‘Implant’ Offers Alternative to Traditional Surgeries
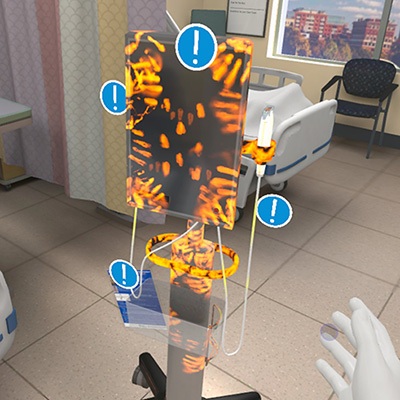
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market
- Philips and Masimo Partner to Advance Patient Monitoring Measurement Technologies
- B. Braun Acquires Digital Microsurgery Company True Digital Surgery
- CMEF 2025 to Promote Holistic and High-Quality Development of Medical and Health Industry

 Expo
Expo
- Imaging Technology Detects Early Signs of Cardiovascular Risk Through Skin
- New Therapeutic Approach Marks Breakthrough in Pediatric Heart Disease
- AI Model Accurately Identifies Prediabetics Using Only ECG Data
- Injectable Disease-Fighting Nanorobots to Improve Precision Cancer Therapy
- Web-Based Tool Enables Early Detection and Prevention of Chronic Kidney Disease
- First-Of-Its-Kind Probe Monitors Fetal Health in Utero During Surgery
- Light-Activated Tissue Adhesive Patch Achieves Rapid and Watertight Neurosurgical Sealing
- Ultrasound Device Offers Non-Invasive Treatment for Kidney Stones
- Minimally Invasive Coronary Artery Bypass Method Offers Safer Alternative to Open-Heart Surgery
- Injectable Breast ‘Implant’ Offers Alternative to Traditional Surgeries
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- WHX in Dubai (formerly Arab Health) to bring together key UAE government entities during the groundbreaking 2026 edition
- Interoperability Push Fuels Surge in Healthcare IT Market
- Philips and Masimo Partner to Advance Patient Monitoring Measurement Technologies
- B. Braun Acquires Digital Microsurgery Company True Digital Surgery
- CMEF 2025 to Promote Holistic and High-Quality Development of Medical and Health Industry